Commentary on the EMA Guideline on strategies to identify and mitigate risks for first‐in‐human and early clinical trials with investigational medicinal products - Gerven - 2018 - British Journal of Clinical Pharmacology -
PDF) Adaptive clinical trial designs for European marketing authorization: A survey of scientific advice letters from the European Medicines Agency
Overview of comments - Requirements to the chemical and pharmaceutical quality documentation concerning investigational medicina
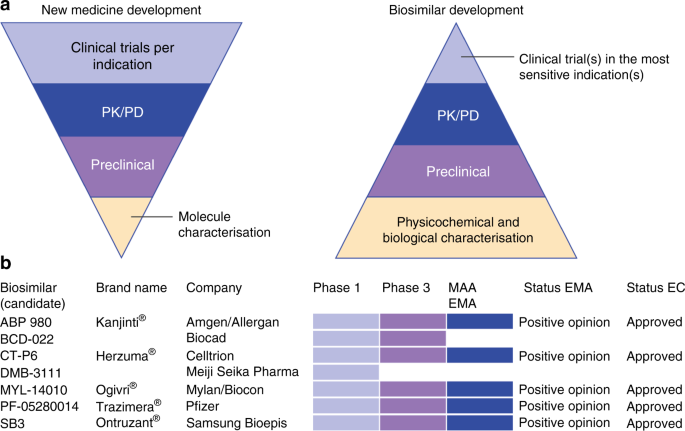
The arrival of biosimilar monoclonal antibodies in oncology: clinical studies for trastuzumab biosimilars | British Journal of Cancer

Tag | CRO, Clinical, TMF, eTMF, trial master file, inspection, audit, audit ready, inspection ready, Clinical Research Organisation, regulation, EU, EMA, MHRA, Andy FIsher | Pharma IQ

When innovation outpaces regulations: The legal challenges for direct‐to‐patient supply of investigational medicinal products - Malone - 2022 - British Journal of Clinical Pharmacology - Wiley Online Library
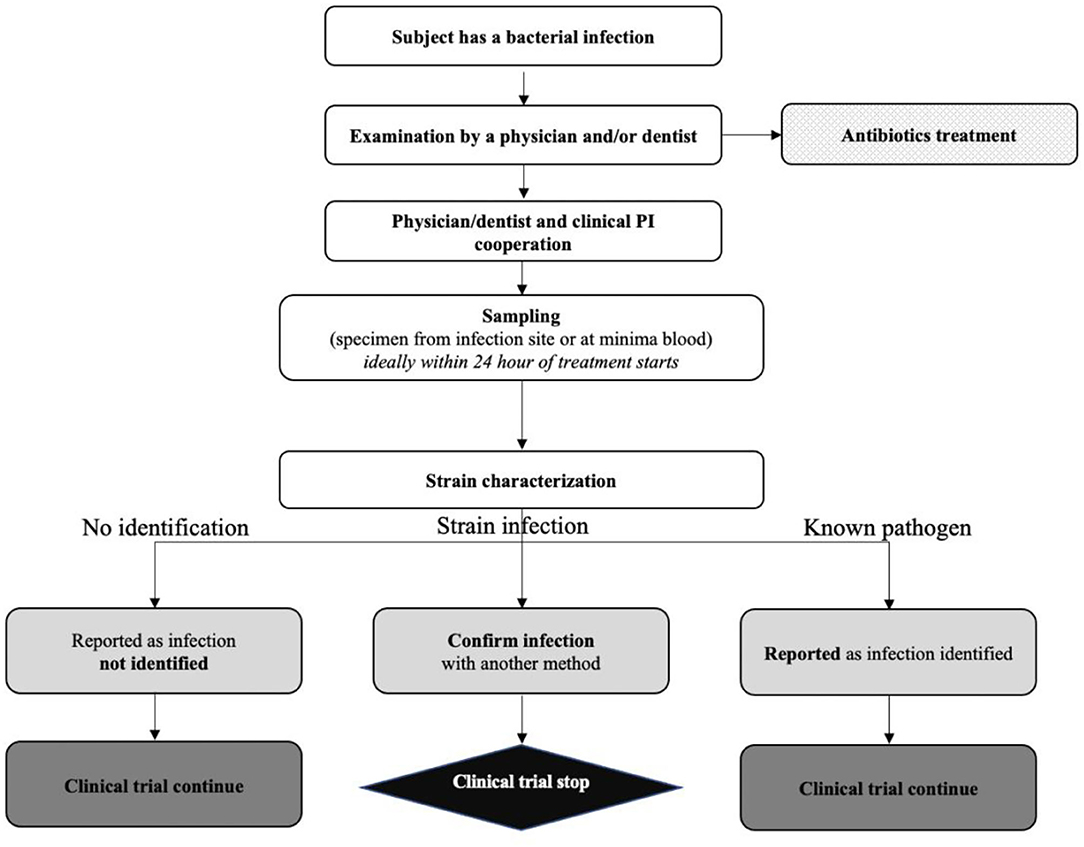
Reflection paper on guidance for laboratories that perform the analysis or evaluation of clinical trial samples

Current landscape of clinical development and approval of advanced therapies: Molecular Therapy - Methods & Clinical Development
Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience